Ever wondered the science behind the structure of the solar system? Let me present you a blog post on Solar System and the Atom. Hope you will like it.
The sun is the largest and the center with eight planets, asteroids and moon orbiting around it. What if I tell you there exists a similar structure in a tiny atom?
Yes! To be honest, we all know that three basic particles namely Protons, Neutrons, and Electrons forms the atom. The nucleus is the center of the atom and the electrons orbit around it, quite similar to the solar system.
Protons are positively charged particles, neutrons are neutral particles and electrons are negatively charged particles. Protons and neutrons together make the nucleus of an atom.
Ernest Rutherford a physicist from New Zealand discovered the atomic structure and proposed it as Rutherford atomic model. It is also known as the planetary model of the atom or nuclear atom. The ‘plum pudding model’ by JJ Thomson failed to explain certain experimental results of the atomic structure so in 1911 Rutherford came up with gold foil experiment to demonstrate the structure of an atom.
In order to test JJ Thomson’s plum pudding model, Ernest Rutherford conducted the experiment along with his companions Ernest Marsden and Hans Geiger. He said if the plum pudding model by JJ Thomson is correct then when a high-velocity alpha particle is passed through gold foil it should pass in a straight line. The experiment was conducted in the dark so that the light of the alpha particles can be observed.
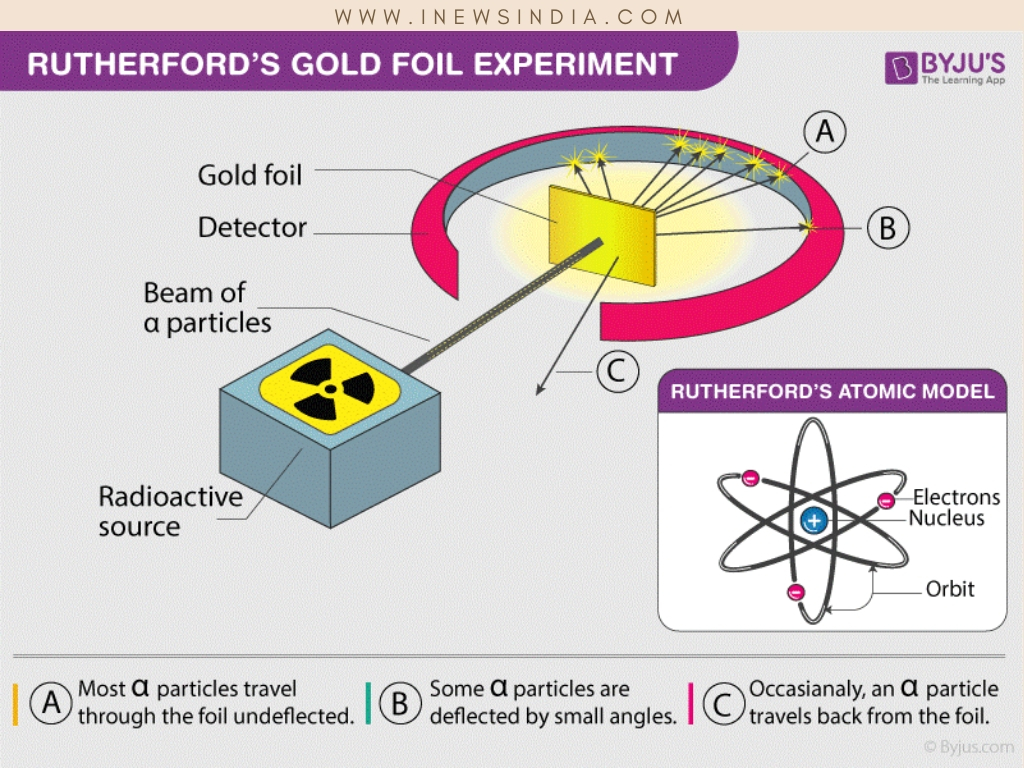
A gold foil of thickness 8.6 x 10-6 centimeters. A beam of high-velocity alpha particles from a radioactive source was shot on the gold foil. Most of the alpha particles passed through the foil in a straight line, undeflected. Some alpha particles deflected from the expected path with small angles. Few traveled in the reverse direction. Therefore he concluded that:
- Since there is a deflection of alpha particles there is a positive charge in the atom which is the nucleus
- Since most of the alpha particles passed through the foil in a straight line the atom is mostly empty space
- Most of the atom’s mass is carried by the nucleus as some alpha particles traveled in the reverse direction
Therefore the atom consists of a positive nucleus and is surrounded light, tiny negatively charged particles called electrons. Since the electrons move around the nucleus same as the planets orbit around the sun, it is called the Planetary Model of Atom.
If you find this interesting and would love to know various other concepts like the plum pudding model by JJ Thomson, different chemical reactions, etc. visit BYJU’S – India’s largest k-12 learning app. An online course where expert faculties conduct classes with interesting 3D/ Graphical images and videos to make you understand the concepts easily.
For a better understanding of chemistry concepts, you can subscribe to BYJU’S YouTube channel (I have been introduced to it by one of the cutest girl) and learn in a more engaging and effective way

Leave a Reply